HOME >>
Chemicals
>> Para Methoxy Phenyl Acetic Acid

|
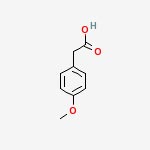
Para Methoxy Phenyl Acetic Acid
|
|
IUPAC Name:
2-(4-methoxyphenyl)acetic acid
Synonyms:
Homoanisic acid, Benzeneacetic acid, 4-methoxy-, p-Methoxyphenylacetic
acid, 2-(p-Anisyl)acetic acid, MOPA, 4-Methoxyphenylacetic acid,
4-Methoxybenzeneacetic acid, (4-Methoxyphenyl)acetic acid,
Acetic acid, (p-methoxyphenyl)-, Acetic acid, p-methoxyphenyl-,
WLN: QV1R DO1, NCIOpen2_000187, (p-Methoxyphenyl)acetic acid,
M19201_ALDRICH, EINECS 203-166-4, NSC 27799, AIDS017837, P-METHOXYPHENYLACETIC
ACID, AIDS-017837, NSC27799
CAS Registry Number: 104-01-8
Molecular Formula: C9H10O3
Molecular Weight: 166.173900 [g/mol]
H-Bond Donor: 1 H-Bond Acceptor: 3
Melting Point : 84-86 °C(lit.)
Boiling Point : 140 °C3 mm Hg(lit.)
Fp : 193°C
Water Solubility : 6 g/L (20 ºC)
TOXICITY
Oral rat LD50: 1550 mg/Kg
STABILITY : Stable under ordinary conditions
|
DESCRIPTION & APPLICATIONS
Phenylacetic acid is a white crystals with a disagreeable odor;
boiling point 262 C; soluble in alcohol and ether. It serves as an
ingredient in perfume to provide honey-like odor. It is found as a
moiety in some alkaloids and plant hormones. It is formed as
catabolite of phenylalanine. Substituted phenylacetic acid molecule
at alpha position and phenylacetate esters can serve as a drug with
a wide variety of effects including anticholinergic, muscarinic
antagonist, antidote to cholinesterase inhibitors or toxins,
cycloplegic and mydriatic. Phenylacetic acid is used to prepare a
nonsteroidal antiinflammatory drug like diclofenac. It is used the
manufacture of penicillin. Mandelic Acid, phenylglycollic acid, can
be produced from phenylchloracetic acid.
4-Methoxyphenylacetic Acid is used as an intermediate for
pharmaceuticals (especially for dextromethorphan) and other organic
synthesis. It is used as a plant growth regulator and herbicide.
SPECIFICATION
APPEARANCE white to off-white powder
ASSAY 99.0% min
CLARITY IN ETHANOL clear
Phenylacetic acid definition - medical
An abnormal product of phenylalanine catabolism that appears in the
urine in phenylketonuria.
Synthesis of Phenylacetic Acid
Phenylacetic Acid from Benzyl Chloride
10% of a solution of 125 g benzyl chloride [1] in 250 ml of
sodium-distilled diethyl ether is added to 24 g magnesium turnings
under 100 ml ether, and a small iodine crystal is added. after start
of the grignard reaction the rest of the benzyl chloride is added
with stirring to maintain gentle boiling (if the reaction becomes
too vigorous, useless 1,2-diphenylethane is formed) and the mixture
is heated and stirred until most magnesium is dissolved. The
grignard reagent solution is poured on 1 kg water-free, crushed dry
ice (solid CO2) and stirred for 2 hrs. 200 ml warm ether is added
and the mixture is heated in a water-bath until the internal
temperature reaches 25°C. 200 ml 32% hydrochloric acid is added, and
the heterogenous mixture stirred until any inorganic precipate is
dissolved. after filtering, the organic layer is separated, washed
with cold water, and dried over 20 g anhydrous sodium sulfate. The
ether is distilled off, and the rest is recrystallized from water,
to yield 75% - about 100g - phenylacetic acid, mp 76-77°C.
Phenylacetic acid from Benzyl Cyanide
In a silica basin of about 35 cm. diameter, a mixture of 2.5 kilos
of benzyl cyanide [3] and 7.5 kilos of 70% sulfuric acid is warmed
until a few bubbles of gas appear. The heating is at once stopped,
since a very vigorous action now ensues. As the vapors evolved are
injurious to health, the basin is covered when the reaction begins
with a sliding cover from which the vapors can be led into water. As
soon as the reaction ceases, the product is poured upon crushed ice
in a large, earthenware vessel. Crude phenyl acetic acid,
contaminated on the surface with phenyl acetamide, is precipitated
on cooling. In order to remove the amide, the acid is dissolved in
lukewarm dilute soda solution. The amide remains undissolved and can
be filtered off, while the phenyl acetic acid goes into solution as
sodium salt and is reprecipitated in a sufficiently pure state by
the addition of dilute mineral acid. After drying it is acceptably
pure.
 Note:
These API/ chemicals are designated as those that are used in
the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT. Note:
These API/ chemicals are designated as those that are used in
the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT.
Note /Government Notification: N/A

|


|


